Therapeutic Oligonucleotides
GMP Oligo manufacturing
& CMC Services
Eurogentec is a leading therapeutic oligonucleotide manufacturer (CDMO) with over 40 years of experience. We offer comprehensive end-to-end solutions for the development and manufacturing of high-quality oligonucleotides for innovative therapies.
Our state-of-the-art facilities and team of experts ensure efficient and reliable production, from preclinical to clinical phases.
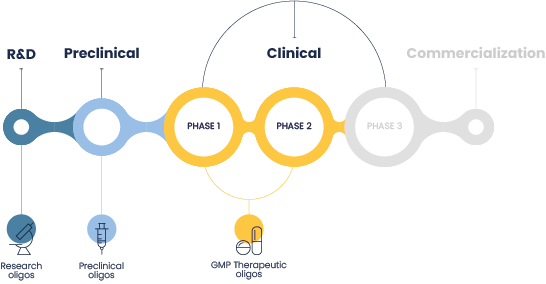
Our expertise in therapeutic oligonucleotide
development & manufacturing
We provide comprehensive and tailored solutions for the development, manufacturing,
and testing of preclinical and clinical oligonucleotides.
We excel in the production of oligonucleotides from research & discovery phases to
oligo screening libraries, and the production of larger quantities needed for preclinical and early clinical studies.
We accompany you from development to clinical phases
| Phases | Research & Discovery | Preclinical | Preclinic to clinic Engg Batch | Clinical |
| Oligo Grades | RUO | RUO | GMP | GMP |
| Examples of Application | Early DD steps incl Screening libraries to lead compound | DD: In vivo & in vitro studies; other high demanding application | GLP tox/safety | Early Clinical Phases |
| Quantity | From micrograms to several grams | From hundred of miligrams to several grams | From hundred of miligrams to several grams | From several grams to hundred of grams |
| Agreements | ||||
| Confidentiality agreement | ✓ | ✓ | ✓ | ✓ |
| Master Service Agreement | On request | On request | ✓ | ✓ |
| Quality Agreement | - | - | ✓ | ✓ |
| Dedicated Project Management | ✓ | ✓ | ✓ | ✓ |
| Quality | ||||
| Quality systems | ISO9001 | ISO9001 | GMP | GMP |
| Incoming Raw Material Specification & testing | - | - | ✓ | ✓ |
| Optimization of analytical methods | - | On request | ✓ | ✓ |
| Equipment/methods qualification | - | - | ✓ | ✓ |
| Validation work | - | - | ✓ | ✓ |
| Classified clean room | - | - | ✓ | ✓ |
| Retain samples | - | - | ✓ | ✓ |
| Stability study | - | On request | ✓ | ✓ |
| Documentation | ||||
| QA documentation | CoA | CoA or Summary batch record |
Summary batch record or BMR |
BMR |
| Change Control Notification | - | - | ✓ | ✓ |
Custom oligo design & chemistries
We specialize in designing and synthesizing a wide range of oligonucleotides, including DNA, RNA, siRNA, miRNA mimics, aptamers and CpG oligos, tailored to your specific needs .
Diverse oligo modifications
Our expertise covers a broad range of chemistries (2'O-Me-RNA, 2'F-RNA, etc.) and modifications (Fluorescent dyes, GalNAc, PEG, lipids etc.) to enhance stability, binding affinity, specificity, and delivery of your therapeutic oligonucleotides.
Oligo types for gene-therapy
We produce different types of oligos such as modified DNA, antisense oligonucleotides (ASO), RNA interference (RNAi).
| ASO | siRNA | Aptamers | miRNA (mimic) | CpG Oligos |
| Antisense oligonucleotides (ASOs) are short synthetic nucleic acids that bind specific RNA to modulate gene expression. They are used to treat genetic disorders, cancer, and viral infections. | Small interfering RNA (siRNA) are short RNA sequences that degrade specific mRNA to prevent protein translation. This RNA interference (RNAi) is used for gene-based therapy to silence genes linked to cancer and genetic disorders. | Aptamers are short, synthetic nucleic acid molecules that fold into specific three-dimensional shapes, to bind tightly and selectively to target molecules. They are used in a wide range of applications, including targeted drug delivery for treatment of cancer, infectious diseases, and autoimmune disorders. | MicroRNAs (miRNAs) are small RNA molecules that bind to mRNA and prevent its translation. They play roles in cellular processes and have potential in treating cancer, cardiovascular disorders, and neurodegenerative diseases. | CpG oligonucleotides are synthetic DNA sequences with unmethylated cytosine-phosphate-guanine motifs that trigger immune responses. They are used to boost vaccine efficacy and in immunotherapy for cancer and infectious diseases. |
High Quality Manufacturing Process
Every step of our extensively proven manufacturing process is thoroughly documented and tested.
Every oligonucleotide we deliver is of the highest quality and efficacy.
For clinical oligonucleotides, we use a validation approach compliant with ICH guidelines.
Our QMS ensures that all activities are conducted systematically to proactively anticipate and
mitigate potential risks, ensuring that our processes consistently produce oligos that meet the expected specifications.
Through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ),
we verify that all manufacturing equipment operates within specified parameters.
This step guarantees the integrity and consistency of our production processes.
Our analytical method validation procedures confirm that our testing methods are accurate, sensitive, and specific.
This ensures that the analytical data we generate is robust, reliable, and suitable for the intended clinical phase of the oligonucleotide.
Each activity is documented in detail. This documentation not only demonstrates our commitment
to transparency but also facilitates compliance with FDA, EMA, and other regulatory requirements.
Deviations are promptly addressed through Corrective and Preventive Actions (CAPA),
and continuous improvement is integral to our manufacturing processes.
Stability studies
We are equipped to perform stability testing on site to determine the optimal storage condition and the integrity of your oligonucleotides.
- Testing performed on both Engineering and GMP batches
-
Standard temperature tested:
- -20°C +/- 5°C,
- 5°C +/- 3°C
- 25 °C ± 2 °C / 60 % RH ± 5 % RH,
- and 40 °C ± 2 °C / 75 % RH ± 5 % RH
- Forced degradation & stress studies
- Format comparison (liquid vs lyophilized format)
- Evaluation at various time intervals
Comprehensive Project Management
Our dedicated project management team ensures every phase of your oligonucleotide manufacturing
project is carefully planned and executed, from initial conception to final delivery.
Our experienced project managers maintain clear communication, act proactively to anticipate
potential challenges and implement strategic solutions for smooth and efficient progress.
Working with Eurogentec
-
First contact
-
Introduction with our specialists
-
Confidentiality Disclosure Agreement (CDA)
-
Project evaluation
-
Quotation
-
Master Supply/Project Agreement
-
Quality Agreement
-
Project start
-
Production
-
Release Drug Substance
Contact Eurogentec Today!
Let us help you bring your innovative therapeutic oligonucleotide therapies to life. Contact us today to discuss your project and receive a quote.