GMP Production
Custom mRNA manufacturing
Your trusted partner in mRNA production, supporting every stage of development. We provide custom mRNA manufacturing services through in vitro transcription (IVT). With deep expertise in mRNA synthesis and purification, we offer tailored solutions from discovery and screening to clinical trials and commercialization.
Accelerate success
with an expert mRNA partner
With nearly a decade of experience in mRNA process development, manufacturing, robust quality
controls (QC), and a comprehensive risk assessment approach, we deliver tailored solutions to
help you achieve your drug development milestones efficiently. Guided by solid knowledge and
expertise, we support our customers at every stage, delivering mRNA in the right quantity and
quality, precisely when it's needed.
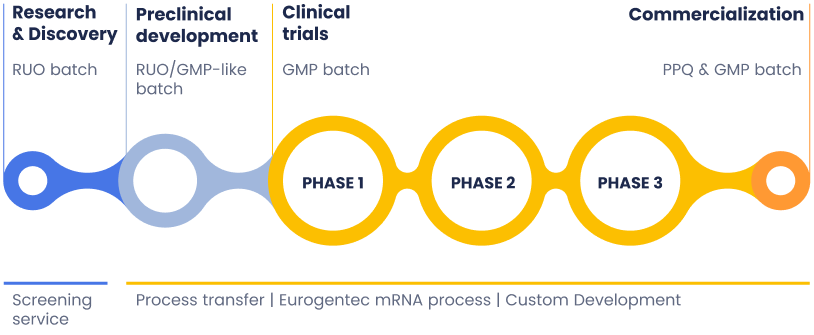
What is included in our mRNA services?
Our mRNA manufacturing service includes process development, plasmid linearization, and scalable IVT production of
mRNA Active Pharmaceutical Ingredients (APIs) using customer-provided or Eurogentec-sourced templates.

Template
Our flexible service supports a variety of template grades and types.
- Customer-provided or Eurogentec-sourced materials
- Includes circular plasmids, linear plasmids, and synthetic DNA
We also offer a plasmid linearization service, streamlining the process to ensure optimal results.

mRNA Manufacturing
Our scalable manufacturing process delivers tailor-made mRNA.
- IVT mRNA manufacturing from 0.1g to 50g
- Incorporation of modified ribonucleotides
- Co- or post-transcriptional modifications, including:
- Capping
- Poly-adenylation
- Purification: precipitation, tangential flow filtration (TFF), and chromatography

Quality
Our downstream process ensures exceptional purity and quality.
- Quality Control: Offering comprehensive in-house QC packages and customizable testing options.
- Quality Assurance: ICH Q7 compliance for GMP RNA drug substance production for clinical trials.
Options for custom mRNA design
Capping
Our services include a wide variety of capping technologies including ARCA (Anti-Reverse CAP Analog) capping technology and other alternatives such as CleanCap, IP-free caps, as well as options for uncapped mRNA, enzymatic capping, and phosphatase treatments.
Sequence
Using customer-provided sequences, we manufacture mRNA with either unmodified or modified nucleotides to suit your project’s specific needs.
Poly(A) Tail
The poly(A) tail can be incorporated directly into the template plasmid DNA or added post-synthesis, providing flexibility to match customer’s requirements.
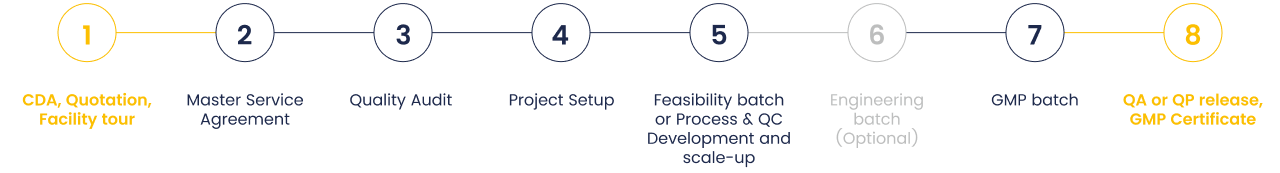
Flexible mRNA manufacturing process
Our proprietary manufacturing processes ensure faster production timelines with consistent efficiency and quality.
We also work closely with customers to develop custom mRNA processes tailored to their unique project needs.
For customers with an existing production workflow, we also offer process transfer services.
Scalable production solutions
We manufacture mRNA in single-use bioreactors of various volumes, ensuring flexibility and scalability to meet diverse project requirements. While we typically use 10-liter bioreactors, we can easily accommodate customer-specific needs with adaptable volume options always prioritizing their timeline and budget.
QC packages tailored to your needs
With a variety of QC packages and testing options, we ensure you can assemble
a tailored QC strategy to meet your specific scientific and regulatory goals.
Comprehensive GMP Quality Testing
Our GMP QC package includes rigorous analyses:
- Appearance
- pH
- Size
- Quantification
- Integrity
- Residual pDNA
- Residual Host Cell Proteins (HCP)
Ensuring mRNA Safety
Our safety package ensures the integrity of mRNA products through strigent testing, meeting the highest industry standards.
This package includes:
- Endotoxin testing
- Bioburden testing
Advanced QC testing
For specialized needs, our optional QC tests cover advanced analyses including:
- Identity
- % Capping
- % Modified nucleotides
- Poly(A) tail length
- Residual enzymes
- dsRNA content
QC Option
We also support the development and transfer of custom QC tests upon request, offering maximum flexibility to accommodate your project.
State-of-the-art facilities
Our facilities, located in Belgium, are designed to meet the demands of both small and large-scale research and GMP-grade manufacturing.
Our 4 GMP suites are equipped for production, purification, and comprehensive QC activities, with robust QA oversight and QP certification processes to ensure every batch meets the highest quality standards.
Adaptable purification strategies
Our capabilities include chromatography, precipitation, and tangential flow filtration (TFF), allowing us to tailor purification methods to specific project requirements. Additionally, we provide the option to work with organic buffers as part of our adaptable purification approach.
Support & project management
Our team of scientific advisors and skilled biochemists is here to guide you in making the best decisions for your current project and long-term needs. We provide tailored recommendations to optimize your mRNA production while guaranteeing full GMP compliance by the end of the development process.
For GMP projects, we assign dedicated project managers who prioritize open and transparent communication at every stage. Their proactive and collaborative approach has been highly valued by our customers, facilitating a smooth and efficient partnership.
Quality management system
Our Quality Management System ensures that every employee operates within the guidelines to produce and deliver GMP RNA drug substance in accordance with ICH Q7.
Each RNA production batch intended to be used in human clinical trials is produced in our GMP facilities accredited by the Belgian ministry of health.
Why choose Eurogentec for your RNA project?
Expertise
Decades of GMP experience with a multiproduct platform.
One single partner
Continuum from clinical to commercial supply.
Project support
Dedicated project managers and scientific advisors.
Fast manufacturing
Proprietary mRNA process for accelerated timelines.
Flexible process
Scalable process and QC packages tailored to your project requirements.
IP-free platform
Proprietary process designed to avoid licensing hurdles.
Related CDMO services
Note: The non-infringement of any patents covering the operation of any process or the use of the product alone or in combination with other for use or sale of the products mentioned above is not warranted by Eurogentec. The customer has the sole responsibility of all and any use of Eurogentec’s products.