Antibody Specialized programs
VHH antibody fragments
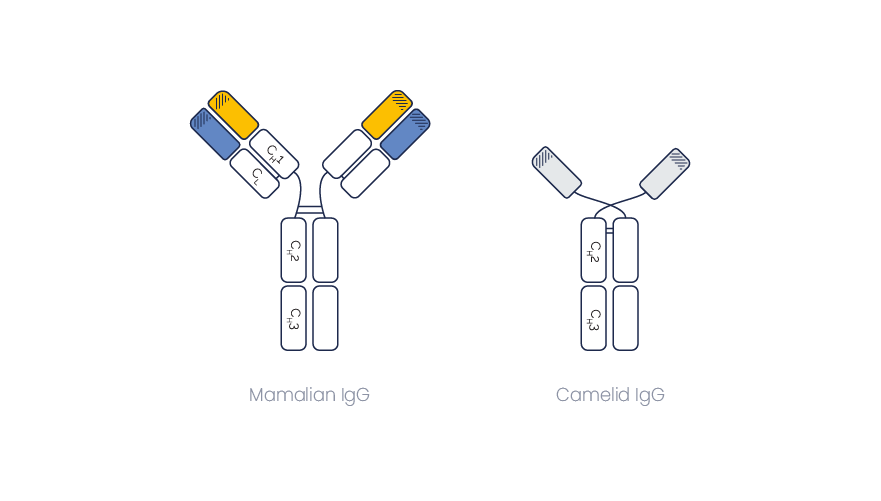
Besides conventional immunoglobulins (IgGs), llamas and other camelids also produce heavy chain-only antibodies, also named single-chain Abs or heavy-chain Abs (HcAbs). They harbor the smallest naturally-occurring antigen binding domain, the VHH Fragment.
Why use Single-chain antibodies?
Immunoglobulins from camelids are sub-classified as IgG1, IgG2 and IgG3.
IgG2 and IgG3 are single-chain Abs, and they both bind the antigen through a unique domain, named VHH (or nanobody®).
They differ by the size of their hinge region which connects the VHH domain to the constant IgG domain (CH2 + CH3).
It results in a size of 90 to 100 kd, instead of ~ 150 kDa for a classical IgG1.
Simpler structure
IgG2 and IgG3 are both devoid of light chains or CH1 domains. Hence their size (~90 kDa) is much smaller than the classical IgG1 size (~150 kDa).
As some disulfide bonds (connecting the heavy and light chains in conventional Abs) are missing.
HcAbs offer a much easier manipulation and a higher stability. HcAbs are e.g. reported to bind antigens at higher temperatures and under more stringent denaturing conditions compared to classical IgGs.
Selectively working with the IgG2/3 serum fraction of an immunized llama is made possible by the availability of anti-IgG2/3 secondary antibodies, which show no cross-reaction toward IgG1.
VHH fragments and their benefits
VHHs have many advantages over traditional immunoglobulin fragments such as scFvs or Fabs.
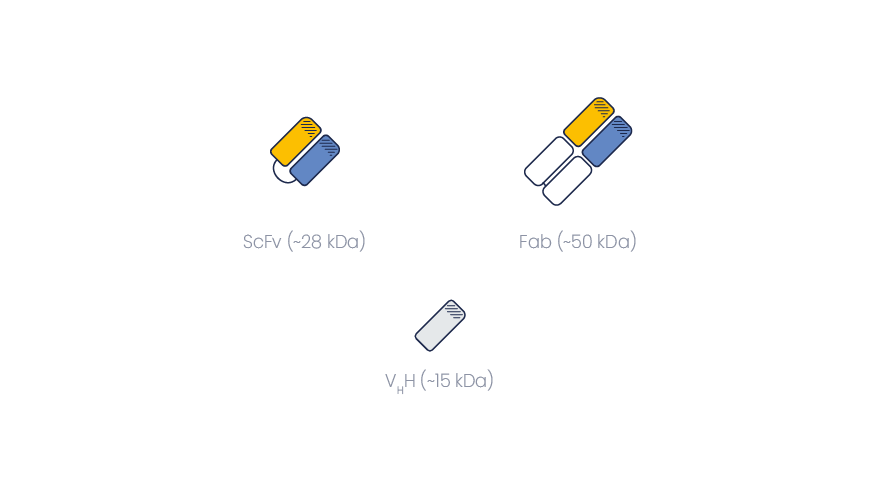
Compared to monoclonal antibody fragments, VHHs are made of one polypeptide chain only. Therefore, they can easily be produced in lower organisms (E. coli, yeast). They also retain full activity compared to the HcAb from which they originate.
On the opposite, Fabs, scFvs and other mAb fragments are made of two polypeptide chains, and their folding without the supporting scaffold of the original mAb may lead to specificity or sensitivity issues. Producing dimeric fragments is also more challenging.